Follow the Calories
We have literally just scratched the surface: we have only talked about what can be observed about calories from outside our bodies. Let’s get into what happens on the inside when we eat food.
First, we need to distinguish two concepts that are often conflated: digestion and metabolism. Digestion is the process by which the body breaks down food in the gastrointestinal (GI) tract and eliminates waste. Metabolism refers to the cellular activities that transform digested food into energy. For this discussion, digestion ends when food is broken down into simple nutrients (glucose, fatty acids, amino acids, etc.) and absorbed into our bloodstream. Metabolism is the subsequent phase when our body synthesizes energy from these inputs.
Our GI tract, from top to bottom, roughly consists of: the mouth, the esophagus, the stomach, the small intestine, the large intestine, the rectum, and the anus. The GI tract is a wonderfully complicated and intricate system. For example, pepsin is a protein-digesting enzyme released by the stomach. Quite thoughtfully, it is only activated by the gastric acid. Once the food moves on from the stomach to the start of the small intestine, the pepsin is neutralized by an alkaline secretion from Brunner’s glands. This mechanism ensures that this protein-eating enzyme will not run amok in the rest of our body, where many parts are made of proteins.
Many of us fail at weight control because we just can’t stop eating. The mechanism by which our body decides we have had enough is complex and not yet fully understood, but it’s not as simple as just having a full belly. Our gut is the largest endocrine (hormone-secreting) organ in the body. As food makes its way through our digestive system, our gut pumps out all sorts of hormones. These hormones are part of a complex messaging system between the gut and the brain. Evidence supporting this interplay is observed in the outcomes of bariatric surgeries. Bariatric comes from the Greek word for weight: baros. Bariatric surgery is just a fancy way of saying weight loss surgery. One type of bariatric surgery works like this: the surgeon reduces the size of your stomach to that of an egg by stapling off a section of the stomach. This newly formed pouch is then directly connected to the small intestine. This way, the food you eat bypasses the rest of the stomach and the upper part of your small intestine.
Two things are surprising about this surgery: First, it works extremely well. Patients lose a lot of weight, fast. And they keep it off. Second, it doesn’t work for the reason doctors thought it would. It was thought that with a smaller stomach, we would feel full with less food. Turns out the stomach is elastic. Not long after the surgery, the small pouch expands back to the size of the original stomach. The real game-changer seems to be the impact on gastrointestinal hormone secretion. After surgery, there’s a spike in several hormones, including one called GLP-1. The weight loss drug Ozempic works by mimicking GLP-1. When food is being digested in the gut, GLP-1 is secreted to signal the pancreas to release insulin in anticipation of the glucose that will enter the bloodstream. The brain perhaps takes this as a sign that we don’t need more food. Because food skips a portion of the GI tract post-surgery, it slides right down to the middle of the small intestine in a less digested state. This changes the mix of hormones the gut sends out, tricking your brain into thinking you’ve eaten more than you actually have.
In the small intestine, glucose, amino acids, and fatty acids enter our bloodstream. Figure 2 is a simplified description of what happens next.
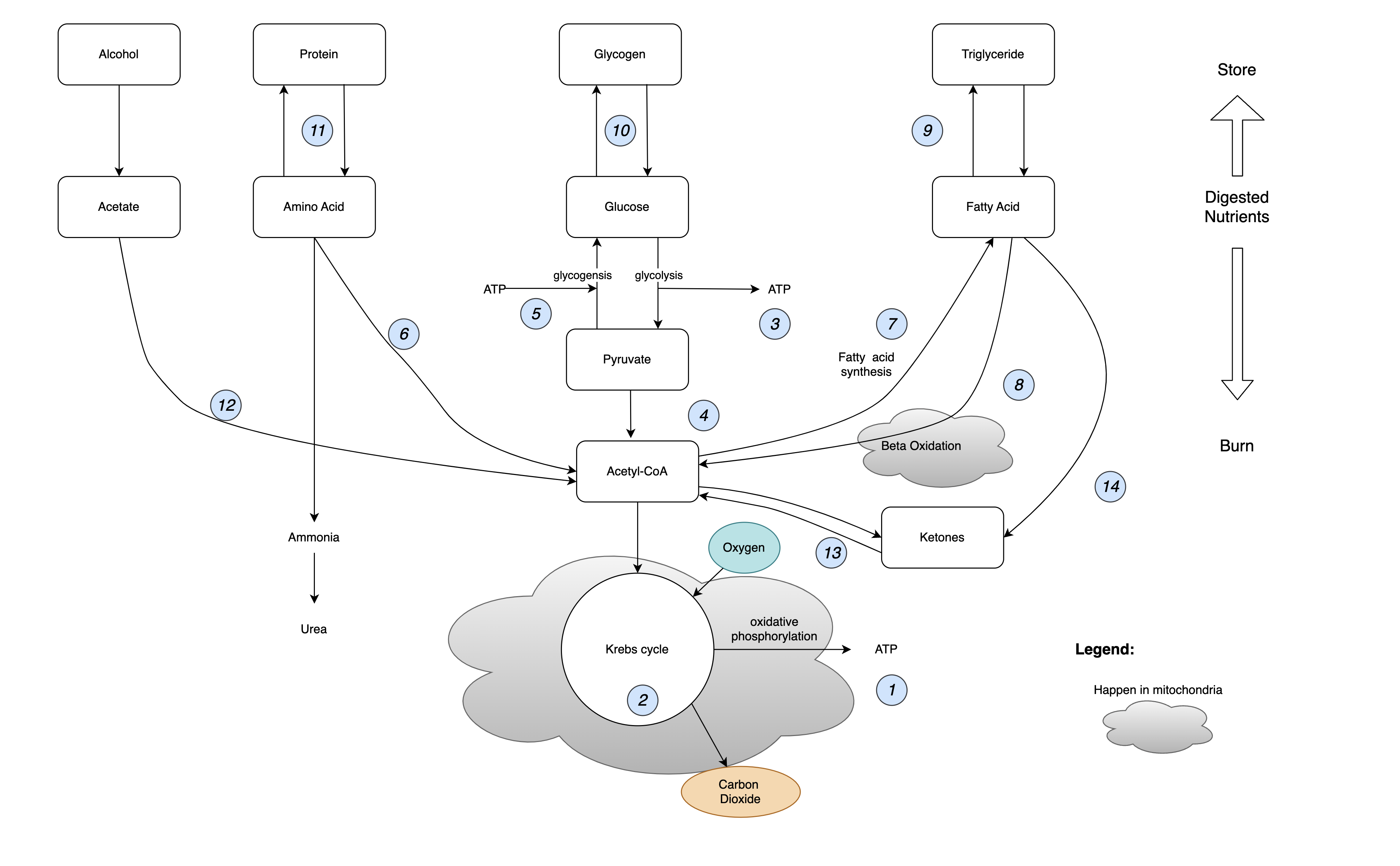
Figure 2. Metabolic Pathways
It looks complicated. But don’t worry, I will now walk you through it:
- ATP, short for adenosine triphosphate, is the energy source for living cells. It acts like a rechargeable battery. An ATP molecule has three phosphoryl groups—hence the prefix “tri.” Energy is stored in the chemical bonds binding the groups together. When a cell needs some juice, ATP breaks one of those bonds, turning a triphosphate molecule into a diphosphate molecule (adenosine diphosphate, aka ADP), and energy is released. ADP can be re-energized to ATP in several ways that we will get into. Remarkably, each ATP molecule is recycled 1,000 to 1,500 times in a single day.
- All life forms on Earth use ATP as their energy storage medium. In plants, photosynthesis generates ATP. In humans, the most efficient mechanism to generate ATP is the citric acid cycle, also known as the Krebs cycle. Figure 3 shows how it works: The bonds between carbon atoms in citrate are broken in the presence of oxygen, producing carbon dioxide, just as it happens in the combustion process inside the calorimeter. The Krebs cycle generates free electrons along with energy, which in turn creates a voltage potential across the inner membrane of mitochondria. This electrical energy is harnessed during oxidative phosphorylation to affix a phosphate group to ADP, thereby synthesizing ATP. Meanwhile, in the other half of the Krebs cycle, the shortened carbon chains are topped up with something called acetyl-CoA, making them long again and ready for another round.
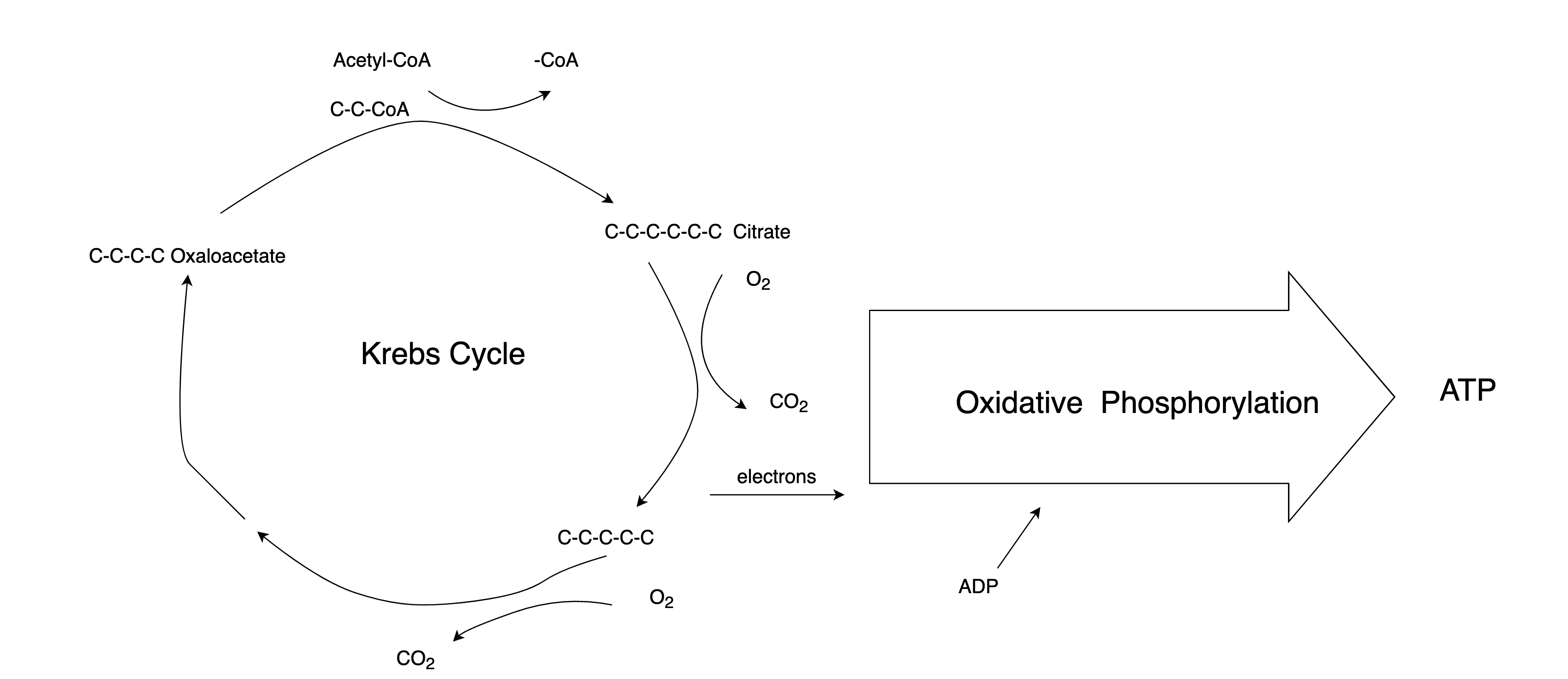
Figure 3. The Krebs Cycle
ATP is not the only product of the Krebs cycle; at least three Nobel Prizes have been awarded for contributions to our understanding of this process
The first step of metabolizing glucose is glycolysis. Glycolysis is the fastest way to generate ATP, and crucially, it doesn’t need oxygen. It’s the energy source for anaerobic activities. Just like a computer has registers, cache, RAM, and a hard disk, the human body has different levels of energy stores that can be tapped at different speeds (I appreciate that the only way this explanation works is if my audience understands computers more than physiology. Don’t get me started on what’s wrong with education :-). As shown in Figure 4, for quick actions, like swinging a golf club, our body uses the ATP that’s already there. After 10 seconds, our body will have to tap the next level of reserve: phosphocreatine. These molecules donate a phosphate group to ADP, making new ATP immediately. When that is used up, the energy released by glycolysis is ready just in time. However, glycolysis can’t keep up for too long. The amount of glucose reserve in our body is limited. Besides, the anaerobic process turns pyruvate into lactic acid, which is the cause of painfully sore muscles.
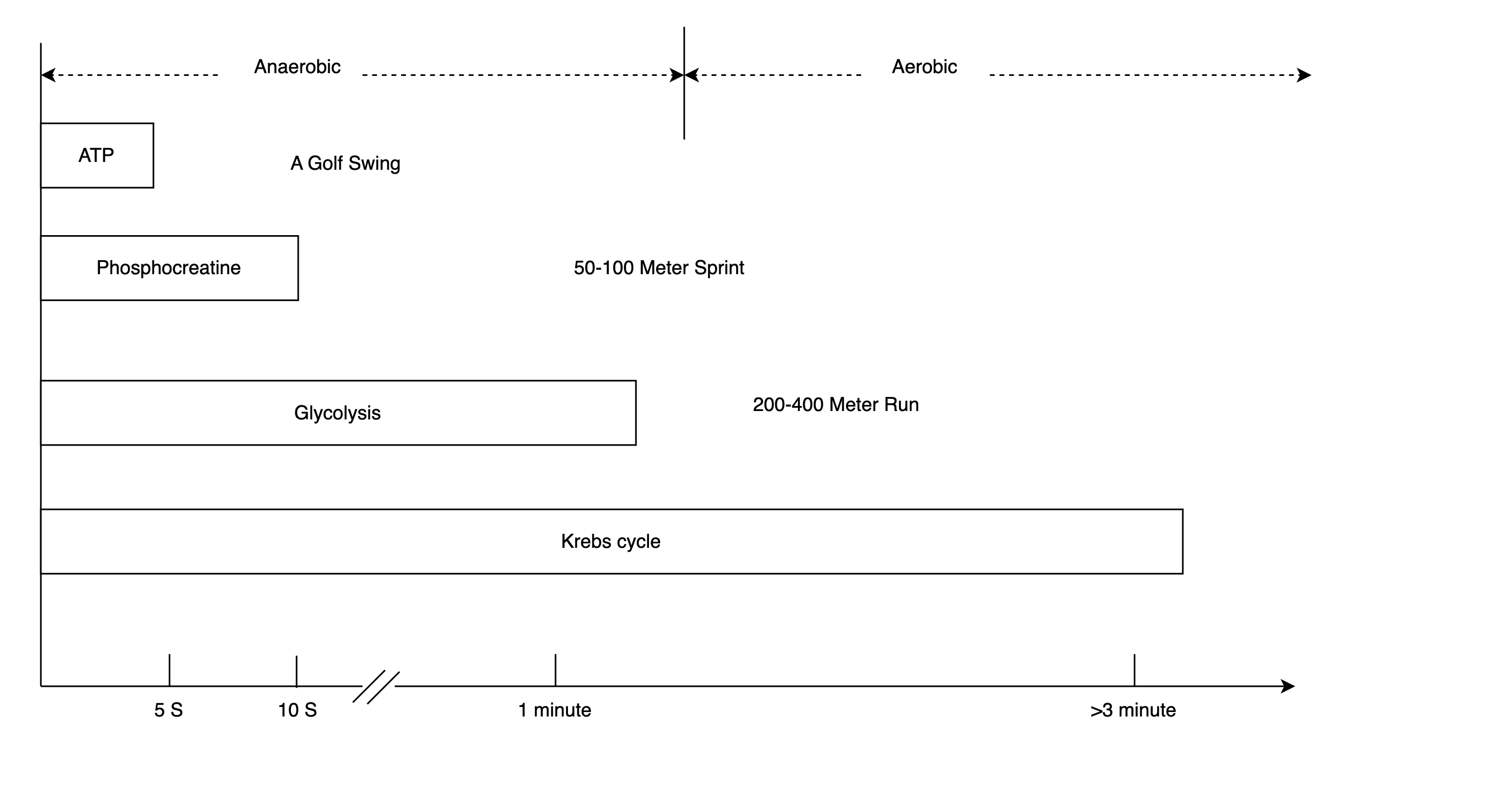
Figure 4. The Energy Caches
The body’s mechanism for enduring energy provision is the aerobic system. It takes a bit longer because oxygen has to get to our muscle cells and start turning pyruvate into acetyl-CoA, which is fed into the Krebs cycle, another step that requires oxygen. The point of heart rate increases during exercise is to speed up the delivery of oxygen by blood. In the Krebs cycle, 28 more ATP are generated, making it our main source of energy for the long haul. By the way, it’s not true that your body doesn’t burn fat if your heart rate is outside the “fat-burning zone.” Upon activation of the aerobic system, the body burns both carbs and fat for energy.
- With a little energy input (a couple of ATP), pyruvate can be converted back to glucose. But acetyl-CoA cannot be converted back to pyruvate. This is important because blood glucose levels are tightly regulated. If it’s too low, the liver will have to make glucose. It can use pyruvate but not acetyl-CoA. This has several consequences that we will talk about later.
On the other hand, if blood glucose levels are too high, insulin is released and triggers cells to take up glucose and convert it into fat. In defense of insulin, it’s not the culprit that makes you gain weight. It does not create the source material for fatty acid synthesis out of thin air: you already have eaten too much to begin with.
- Our body can store a lot of fat. It can store a little bit of glucose. But it has no way to store excess protein. It uses proteins to build and repair body parts like muscles, organs, and even our fingernails and hair. It also uses proteins to make hormones and enzymes. If there’s any protein left over that the body doesn’t need right away, it undergoes the process of deamination, wherein the amine group is removed, and the remainder is converted into acetyl-CoA.
- Acetyl-CoA is the fuel for the Krebs cycle. It’s where protein, fat, and carbs converge in the metabolic pathways. As far as the Krebs cycle is concerned, it doesn’t matter where the acetyl-CoA comes from. If there is too much of it, the only exit is fatty acid synthesis. This is how too much food turns into too much fat. Without portion control, neither an all-meat diet nor a vegan diet automatically makes you lose weight. They sometimes work probably because their participants just lose their appetite due to the limited food choices.
- Fatty acids, with their long hydrocarbon chains, go through the relatively lengthy process of beta-oxidation to become acetyl-CoA. A typical molecule of fatty acid can generate 106 ATP when fully metabolized with oxygen. In contrast, each molecule of glucose generates 30 ATP. Pound for pound, fat is the body’s most efficient storage medium for energy.
- Fat is stored as triglycerides in fat cells, or adipocytes. The fat cells are like balloons in their capacity to expand. When your body accumulates more fat, the number of adipocytes doesn’t really increase, just the size of each one. As long as they still have spare capacity, it’s not dangerous to have a little more fat. How much these cells can stretch before it becomes a problem varies from person to person, mostly because of our genes. For example, the adipocytes of individuals of Caucasian descent are capable of greater expansion in comparison to those of Asian descent, thereby allowing for more considerable safe storage of fat.
- With blood glucose levels stable, the body stores extra glucose as glycogen in the liver and the muscles. This glycogen can be turned back into glucose and provide our muscles with energy at a moment’s notice. Interestingly, that means muscles have everything necessary for the Maillard reaction: protein and sugar. This is most obvious when you pan-sear a scallop on its own and get a nice brown crust.
There are about 400 grams of glycogen stored in the muscles and 100 grams in the liver. If we only use glycogen for energy, all of it will be used up in a day. In contrast, our body fat has enough energy to last us for up to three months.
- All proteins are built from 20 amino acids, just like all English words are built from 26 characters. When we eat proteins, our body dismantles them into their constituent amino acids. Then, it uses them to make new proteins. The proteins we eat are broken down into their building blocks. Nine of the 20 amino acids are so-called essential amino acids because they cannot be synthesized by our body. We have to get them from food.
- Alcohol, as Mr. Atwater pointed out, is also an energy source. It’s eventually metabolized to acetyl-CoA and fed into the Krebs cycle. So, getting a “beer belly” isn’t really about drinking too much beer specifically; it’s about eating or drinking too many calories in general. However, too much alcohol presents a unique hazard: it inhibits fatty acid oxidation and causes fatty liver.
- Sometimes the Krebs cycle cannot take in more acetyl-CoA. To figure out why, let’s revisit Figure 3 and look into the details of the Krebs cycle. We mentioned before that if our blood sugar gets low, the liver starts making glucose from pyruvate. Another precursor for glucose is the glycerol part of the triglycerides. Triglyceride (fat) is three fatty acids attached to a glycerol, as shown in Figure 4. The fatty acid chains cannot be used to make glucose by the liver, so eventually, the liver will need to look elsewhere for raw materials. When both glycerol and pyruvate are used up, the liver begins to commandeer oxaloacetate for gluconeogenesis. Without enough oxaloacetate, the Krebs cycle in liver cells slows down. Surplus acetyl-CoA is converted to ketones and sent elsewhere.
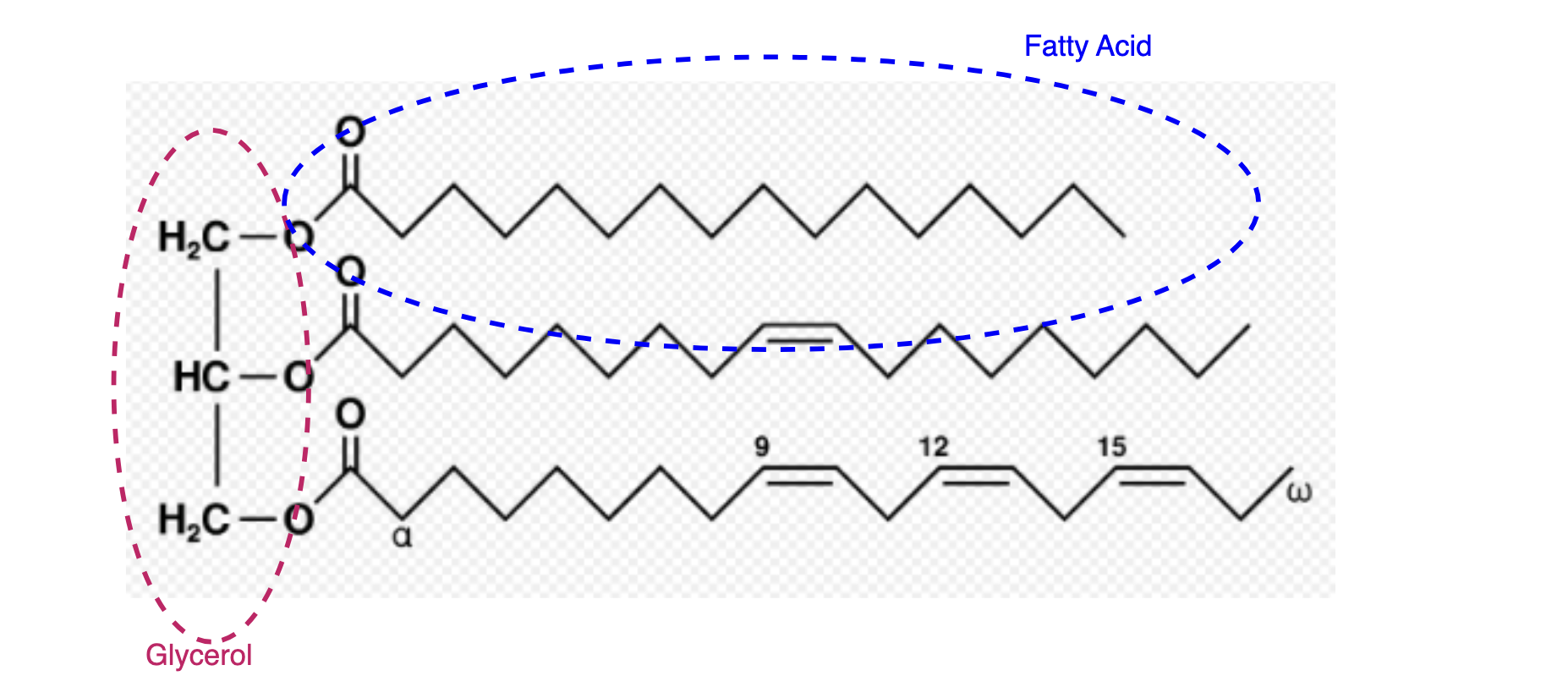
Figure 4. Triglycerides
- Low glucose levels in the body can happen for a couple of reasons. Starvation is one, but these days, it’s more likely due to low-carb diets. Physiologically, the low-carb diet mimics some aspects of starvation. With minimal carb intake, the body adapts to use fat for fuel. Fatty acids are broken down into ketones and sent to cells. The ketone bodies are then taken up by mitochondria, which convert the ketones back to acetyl-CoA and feed it into the Krebs cycle. The liver is the only organ that can divert oxaloacetate to gluconeogenesis. Other cells in the body have no problem metabolizing acetyl-CoA and closing the Krebs cycle.
The situation we just described is ketosis, the state the keto diet tries to induce. This adaptation takes time, though. During intense exercise, your body may run out of glucose reserves before it switches to burning fat for energy. This is known as “hitting the wall” or the “bonk.” It’s for this reason that marathon aid stations provide snacks rich in carbs, to forestall this depletion.