Building blocks for food
To truly understand the effect of temperature on food, we have to go down to the molecular level. There are four basic food molecules: water, fat, carbohydrate, and protein. The water molecule consists of one oxygen atom and two hydrogen atoms. An atom has a nucleus with electrons running circles around it. The orbits of electrons cannot be pinpointed like the orbits of the Earth around the sun. Scientists can only calculate the probability of an electron's location within the atom. The Schrödinger equation is one of the key mathematical tools used for this purpose. I recall encountering this equation in my college physics textbook. The authors declined to show the equation because “the math is too advanced.” I swore if I ever wrote a book, I would not leave my readers hanging like that.
Here’s one special case of the Schrödinger equation:
Well, it may not be entirely unwise to omit it.
Conceptually, you can imagine the region around the nucleus where the electron is likely to be found as a cloud. When atoms come together to make molecules, the electrons from different atoms must share their cloud spaces, akin to two bubbles merging into a new, single bubble. This newly formed bubble is not the sum of the original bubbles; it is an entirely different entity. Chemists call this interatomic linkage a covalent bond. These bonds, which involve pairs of electrons, assume various shapes depending on their energy levels—some resemble spheres, others resemble dumbbells, and still others resemble cloverleaves. How these bonds are formed dictates the chemical and physical properties of the resulting compound.
It seems odd to bring up electron clouds and quantum mechanics in a discussion about cooking, but bear with me; these principles underpin many culinary phenomena. Consider this example: when you eat a hot chili, what do you do to relieve the burning sensation in your mouth?
Hot is not a taste like sweet, sour, or umami. Chilis contain a chemical called capsaicin, which triggers the same sensors in our mouth that sense pain and heat. Our normal reaction is to reach for ice water. But it doesn’t work because the root cause is not heat. Capsaicin does not dissolve in water and get washed away because the chemical bonds in capsaicin are formed differently than those in water (technically, one is non-polar and the other is polar. See The Science of Water about what polar water molecules mean). What works? Milk, for instance. Milk fat does a good job of dissolving capsaicin.
The other three food molecules—fat, carbohydrate, and protein—are all organic compounds. Organic compounds are built upon a carbon backbone. Each carbon atom has four electrons on its outer layer. Each of these electrons wants to pair with an electron from another atom and form a bond. A carbon atom will not be happy until it has formed four bonds, because that’s the state where it has the lowest energy. The simplest organic compound is methane, where the carbon atom forms four bonds with four hydrogen atoms. Butane is the fuel in the blow torches commonly used by home cooks to make crème brûlée. It has a chain of four carbon atoms.
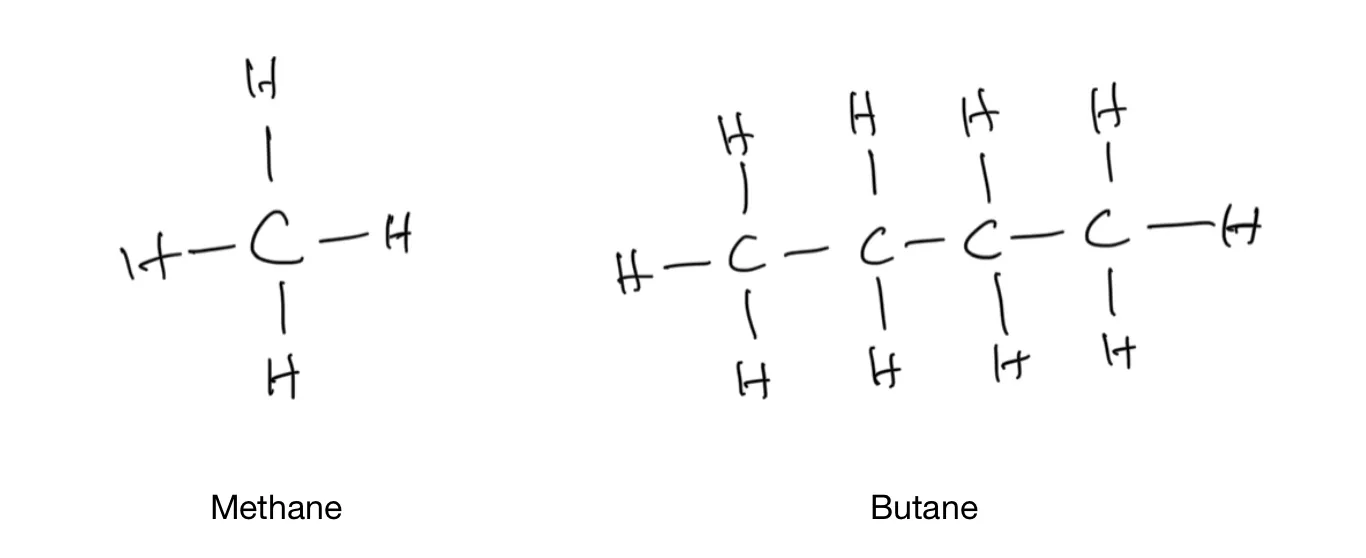
Some simple organic compounds
It is tedious to draw all the little dots for electrons and all the hydrogen atoms and carbon atoms for complicated organic molecules. A common way to simplify the drawing is to:
- Use a short line segment to represent a bond.
- Omit hydrogen atoms and all the hydrogen/carbon bonds.
- Stop drawing the carbon atoms. They are represented by a bend and a black dot in the carbon chain.
Let us now examine the chemical structure of the three macronutrients: Fat, Protein, and Carbohydrate. Understanding these structures will also illuminate how they respond to the application of heat during cooking.