The Phase Change of Water
Intuitively, it’s quite obvious why applying pressure to a substance changes its freezing point: the enthalpy of the system increases, the entropy of the system decreases, which changes the Gibbs free energy…OK, maybe it’s not so obvious. Fortunately, you don’t have to take a class in thermodynamics to understand the phase diagram of water, which contains all the useful information for cooking.
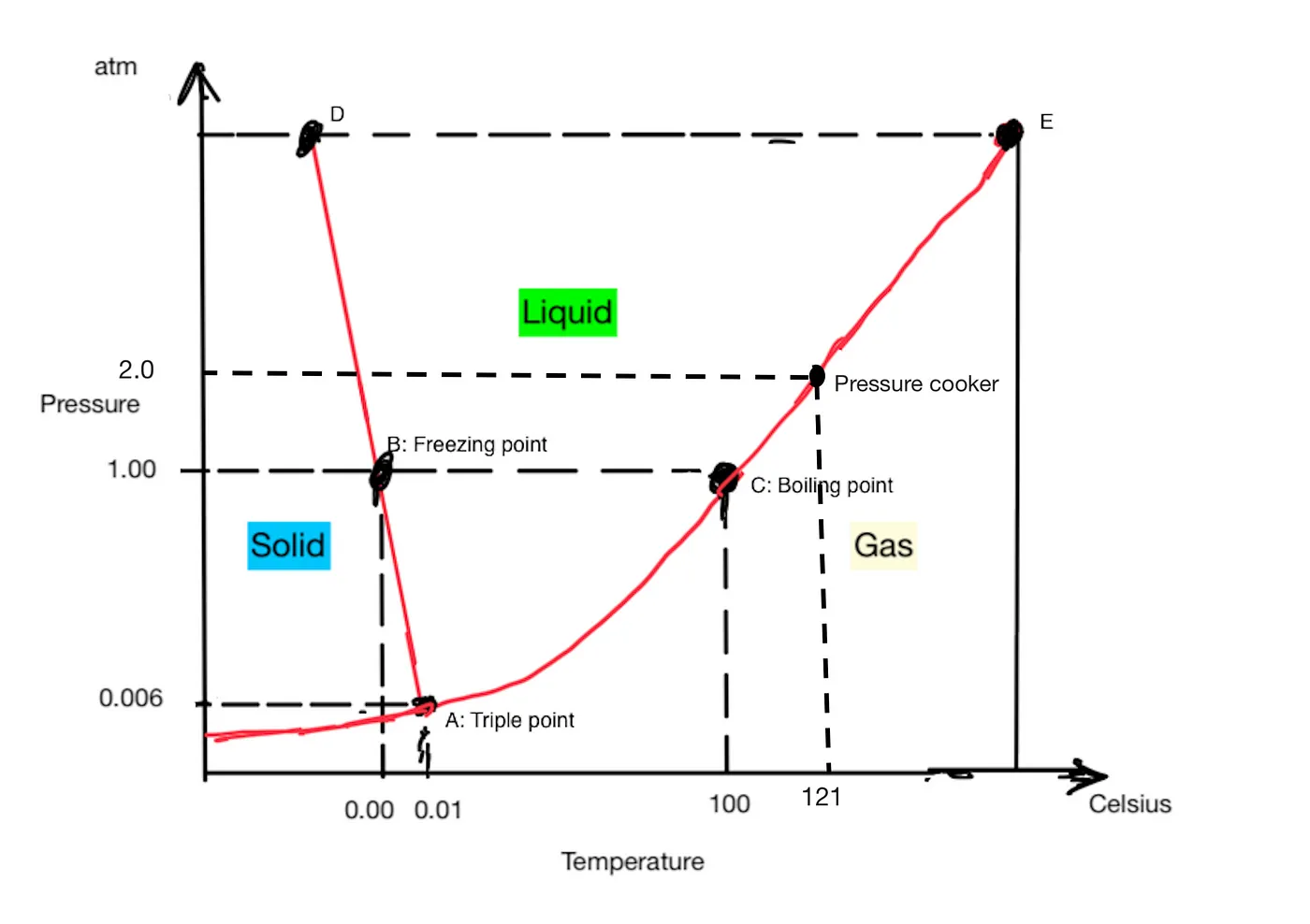
Figure 1: The Phase Diagram of Water
This diagram shows what state water is in at any temperature/pressure combination. The unit of the pressure is atm: the standard atmospheric pressure. Below the AE line, water is gas. Between AE and AD, water is liquid. Left of the AD line, water is solid. Water is special in that the AD line has a negative slope. At 1 atm and 0°C, if pressure is increased, water contracts and turns from solid into a liquid. In the phase diagram of most other substances, the boundary between solid and liquid has a positive slope, as in the phase diagram of nitrogen (Figure 2).
A few points of interest on the phase diagram of water are: C is the boiling point: 100°C at 1 atmospheric pressure. A is called the triple point, where the three phases (gas, liquid, solid) of water exist in equilibrium.
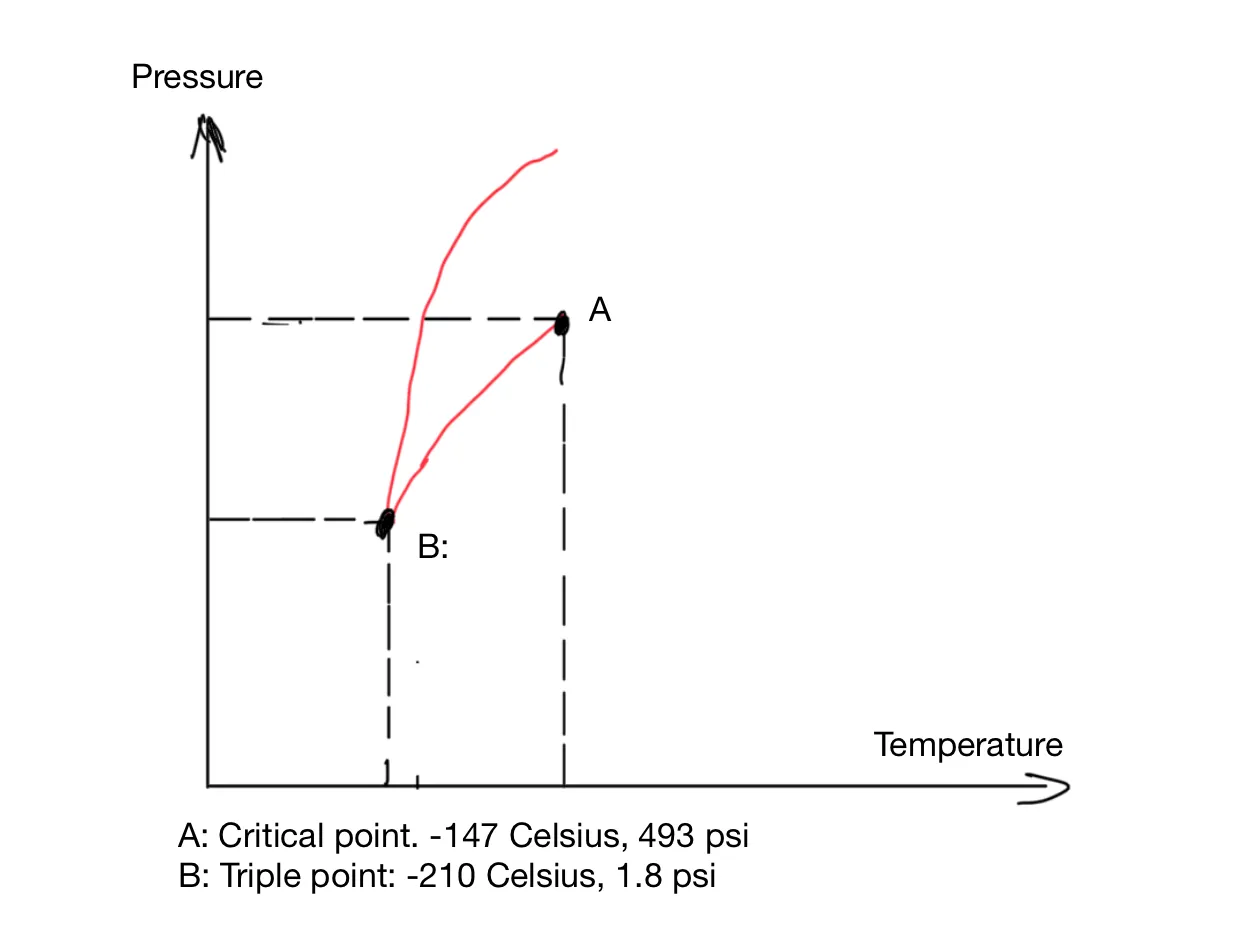
Figure 2: The Phase Diagram of Nitrogen
With the knowledge of the phase diagram, we can play different tricks with water. The tool home cooks are most familiar with is the pressure cooker (aka instant pot). In a typical pressure cooker, the pressure is raised to 2 atm/30 psi, under which the water boiling temperature becomes 121°C. At this temperature, you can soften potatoes for mashed potatoes in about 1/3 of the time.
Normally, when we dry food, water evaporates from the surface of the food. Water inside the food moves to the surface in liquid form, damaging cells and membranes along the way. If we could find a path in the phase diagram where ice turns into gas, without becoming liquid first, we can avoid the damage. Not only is the texture preserved, but also flavor loss is minimized because evaporating water vapor takes few flavor molecules along with it. The process of freeze-drying is so gentle that it’s widely used to preserve flowers.
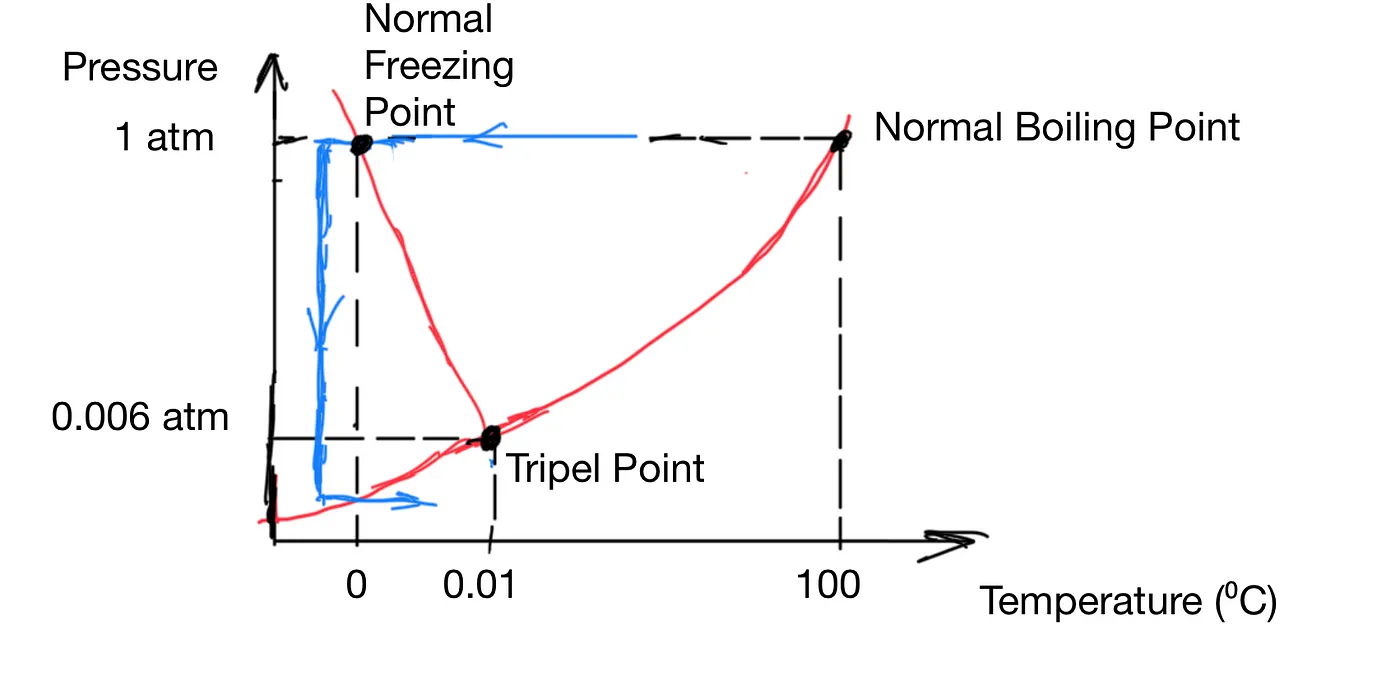
Figure 3: Freeze Drying
The blue path in Figure 3 illustrates how it can be done. Freeze-drying is a tricky process. The food has to be first frozen quickly to avoid damage during the freezing process. Then the pressure is dropped to about 6 mPa, and the food is heated slowly to let the ice sublimate. The heating has to be slow because if vapor pressure builds up in the freeze dryer, all of a sudden, you are at a different point in the phase diagram, and water becomes liquid.
By the way, sublimation doesn’t just happen at low pressure. It also happens at normal pressure. Freezer burn is due to sublimation. Evaporation happens when a water molecule gains enough energy to break free. It doesn’t know it’s in an ice lattice, or it’s in the freezer, or what pressure it’s under. As long as the temperature is not absolute zero, there is always a non-zero chance some molecules will gain enough energy.
Expensive sushi-grade fish needs to be frozen, and frozen well, on fishing boats, not only for preservation but also for killing parasites. The Japanese sushi industry seems to incubate a lot of interesting innovations in freezing technology. One flash freezer uses an electromagnetic field to control the growth of ice crystals, another creates special airflow so that the cold front hits all surfaces of the food simultaneously.
The phase diagram reveals two methods for transitioning water from the liquid to the solid phase: by either lowering the temperature horizontally or reducing the pressure vertically. The rate of temperature change is contingent on the heat transfer process, which typically occurs gradually. In contrast, pressure changes can occur at the speed of sound. Theoretically, if we raise the pressure, water can remain liquid below 0°C. If the pressure is then suddenly reduced, the liquid water rapidly solidifies, forming uniformly small ice crystals. This process is represented by the blue path in Figure 4. Experimentally, this method has demonstrated superior preservation of the texture of potatoes and tofu compared to blast chilling. Although commercial equipment for pressure-shift freezing is not yet available, ongoing research suggests potential future developments.
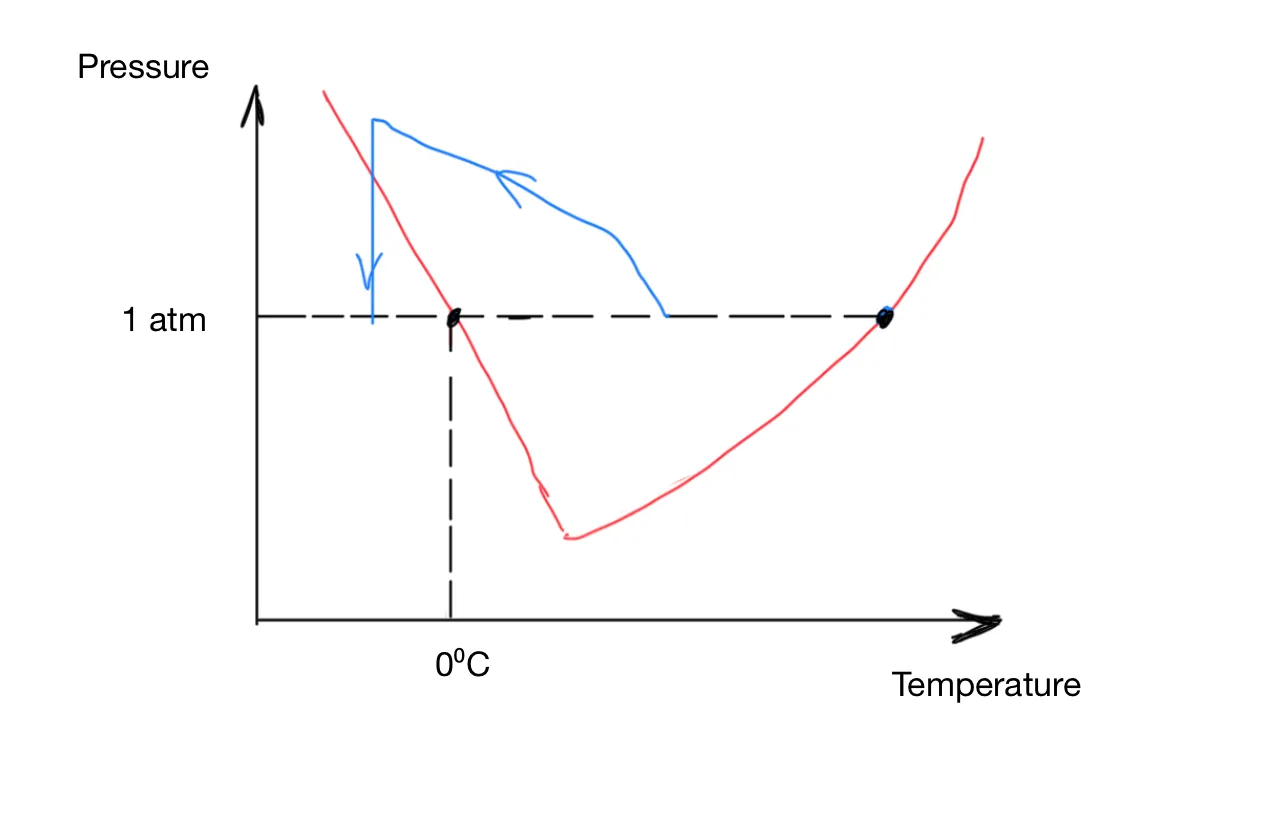
Figure 4: Pressure Shift Freezing
Understanding the science behind common phenomena can lead to magical solutions like pressure shift freezing. There are tremendous opportunities in applying well-known scientific principles to solve real problems for cooks.
Ideas for a Better Fridge
No, we are not talking about the abominations that pass for "smart" fridges, where the door is turned into an oversized iPad. If a screen is to be included on a refrigerator, it should display not the external temperature and humidity, but the conditions inside the fridge itself—and at multiple points within, rather than just a single measurement.
As previously discussed, it’s important to freeze food quickly. While the typical home freezer is adequate for storing frozen goods, it is highly inefficient for the initial freezing process. There should be a dedicated blast chiller chamber. When it's not used as a blast chiller, it can be a regular cold chamber.
The point of the blast chiller is not low temperature, but rapid temperature drop. Blast chilling is not just for making ice cream and freezing fish for sushi. It’s also essential for food safety. As mentioned, the temperature range between 5°C and 55°C is a danger zone for bacterial growth. Allowing hot food to remain in this range for too long can be hazardous. Placing hot food directly into a refrigerator exacerbates the problem, as it can raise the temperature inside the fridge, potentially endangering other items.
In professional kitchens, stringent guidelines dictate that food left in the danger zone for more than four hours must be discarded. Department of Health guidelines state that to safely blast chill food, its temperature must be reduced from +70°C to +3°C or below within 90 minutes. The CDC reports that improper cooling (including improper cooling in the fridge) is, by far, the number one cause of bacterial growth leading to foodborne illnesses. There is also a culinary benefit: a quick chill thickens and gels meat juices before they have a chance to leak.
However, the challenge is not limited to rapid cooling. Improper thawing can similarly expose food to the danger zone for extended periods. The fundamental issue with thawing is that ice conducts heat more efficiently than water. During freezing, the outer layer of food turns to ice first, enhancing heat conduction. Conversely, during thawing, the outer layer liquefies first, slowing down heat transfer. A blast chiller, with its precise control of heating and cooling cycles and accelerated airflow, can also be employed in reverse to expedite the thawing process safely.
It may even be possible for the chiller to incorporate pressure shift freezing technology. As a large appliance equipped with a power compressor, the fridge is already well-suited to manipulating pressures. With the addition of an edge sealer, the appliance could double as a vacuum sealer. Add a scale, a camera, an RFID tag in the reusable bag, and some image recognition algorithm, even the most indifferent cook could have detailed, traceable information for every item stored in the freezer, without the need to manually label anything.
Speaking of lazy cooks, when are we going to get a foot-operated fridge door?