Fat
Structurally, fat consists of three fatty acid molecules attached to one molecule of glycerol, hence the name triglyceride.
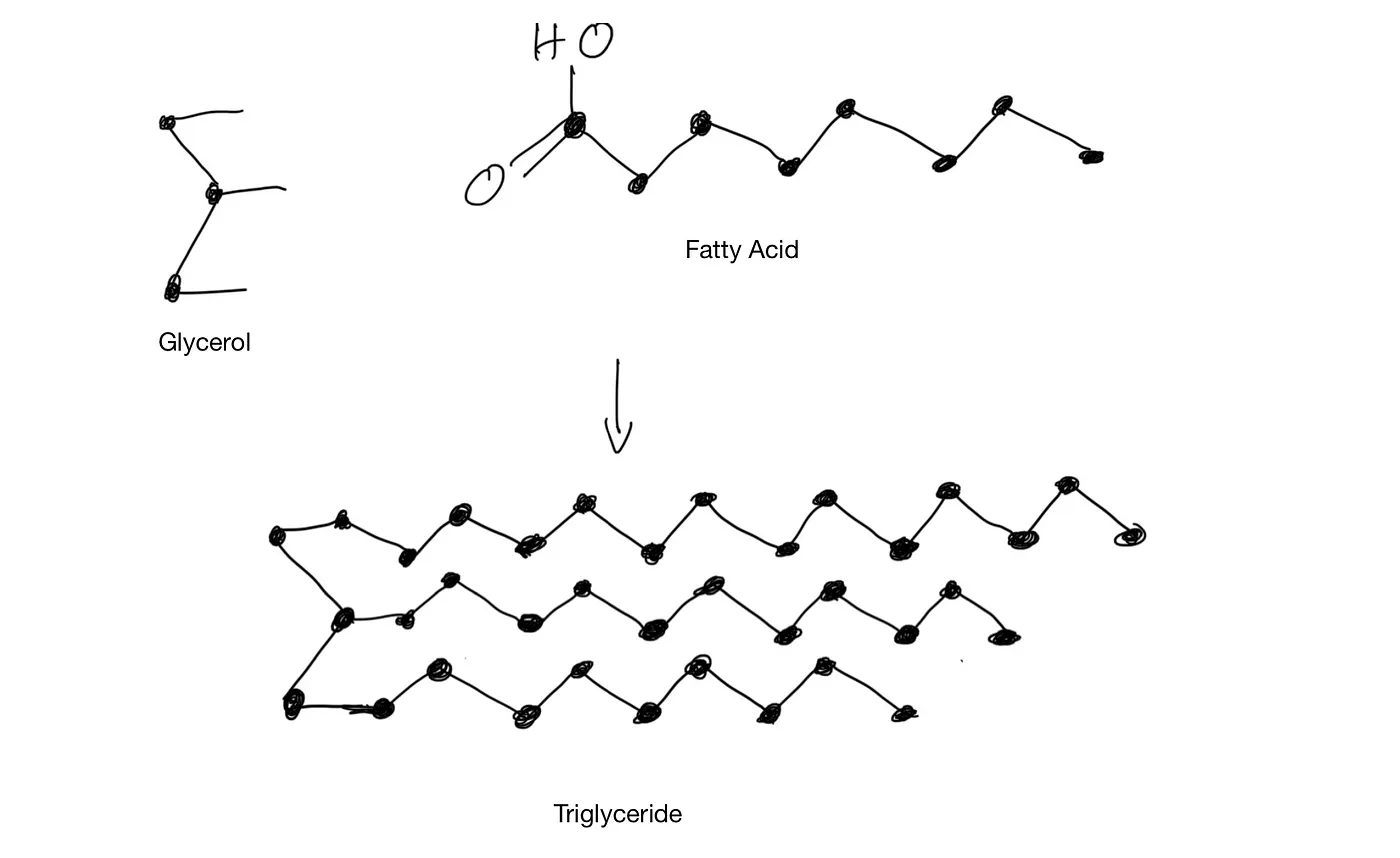
Fatty Acid and Triglyceride
Triglyceride molecules are non-polar. That’s why the non-polar capsaicin dissolves in milk fat.
On the carbon backbone of the fatty acid, if two adjacent carbon atoms share only one pair of electrons, we say they have a single bond. If they share more than one pair of electrons, they have a double bond or triple bond. When they have more than one bond, the extra bond(s) could, in theory, break up and each side would bond to a hydrogen atom, respectively. If all the bonds on the backbone are single bonds, the fatty acid is saturated. If only one bond is a double bond, it’s a monounsaturated fatty acid. If more than one bond is a double bond, then it’s a polyunsaturated fatty acid.
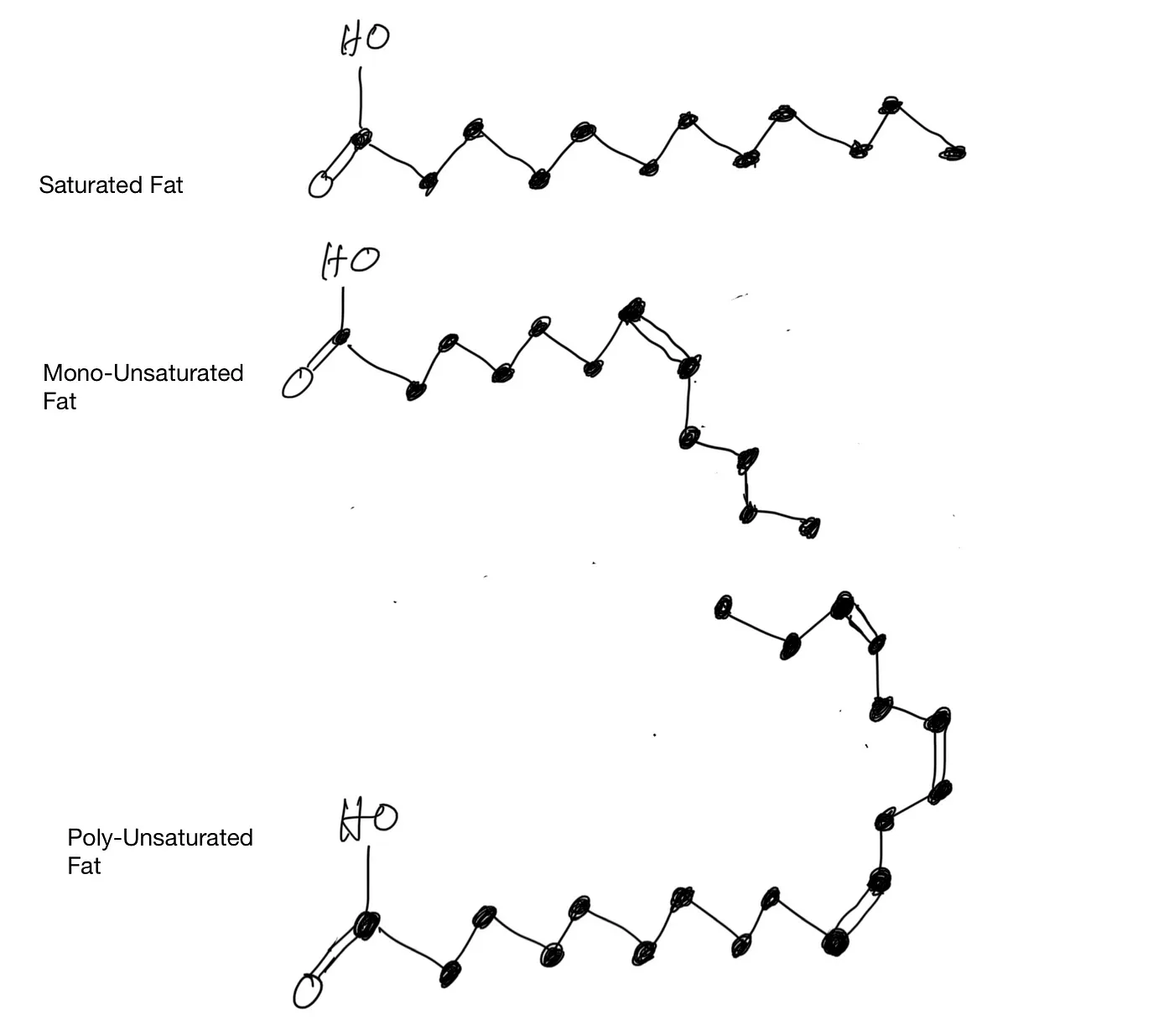
Saturated Fat and Unsaturated Fat
The saturated fatty acids can be packed tightly together in space. But whenever there is a double bond, there is a bend in the chain of carbon atoms. The reason is this: In a single bond, only one pair of electron clouds needs to merge. But in a double bond, two pairs of electron clouds have to merge and coexist in the same general neighborhood between two nuclei. To make matters worse, these electron clouds have different shapes. This quantum game of Twister results in extra space between molecules of unsaturated fatty acids to accommodate the bend. That’s why molecules of unsaturated fat are farther apart from each other.
Because the molecules of saturated fats can be packed together tightly, there is greater intermolecular attraction. Therefore, it takes more energy to separate them, hence higher melting points. Coconut oil is solid at room temperature, while olive oil is liquid, because a higher percentage of coconut oil is saturated fat.
Cocoa butter, the fat in chocolate, exists in one of six possible crystalline forms, depending on how its fatty acid chains are packed together. Only phase 5 and phase 6 have melting points between 28⁰C and 37⁰C. The art of making great chocolate is to precisely control the temperature profile of the process so that only the right crystals will form. That’s how M&M’s “melt in your mouth, not in your hand.”
In naturally occurring unsaturated fat, the hydrogen atoms on either side of a double bond exist on the same side of the bond, as in the left side of the figure below. When hydrogen atoms are forced onto the carbon backbone of vegetable oil to make margarine, some double bonds are changed into single bonds. The remaining double bonds “flip” so that the hydrogen atoms are on opposite sides of the double bond, as in the right side of the figure below. Chemists use the words cis and trans, which in Latin mean “this side of” and “the other side of,” respectively. That’s where the name trans fat comes from.
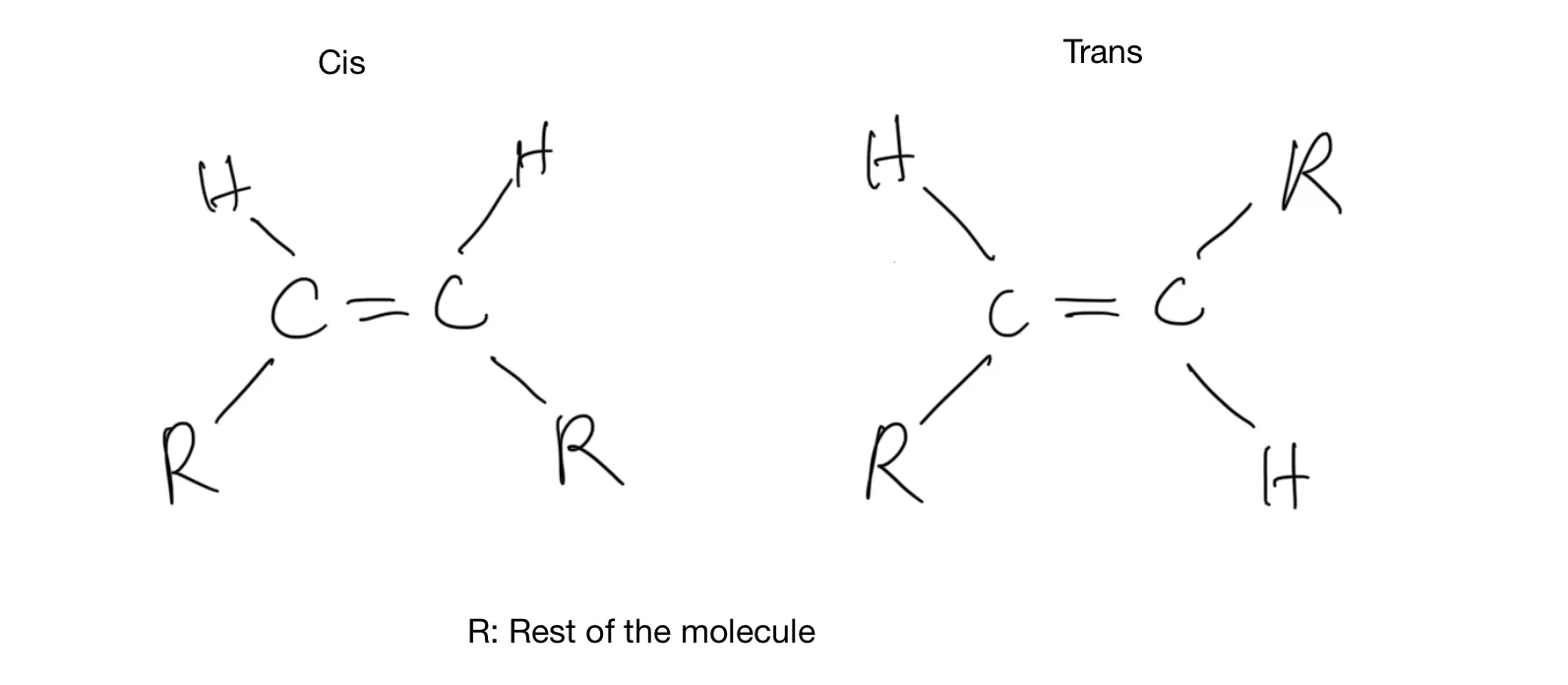
Cis and Trans