Calories? What Calories
In physcis, a calorie is the amount of energy needed to raise the temperature of 1 gram of water from 14.5 degrees Celsius to 15.5 degrees Celsius.
It’s a little more than what you are used to, isn’t it? he specific heat of water depends on its condition. In the isobaric (constant pressure) condition, which is our usual condition, water’s specific heat in the liquid phase is higher at both 0 degrees Celsius and 100 degrees Celsius and dips slightly at 15 degrees Celsius. In the isochoric (constant volume) condition, the specific heat vs. temperature curve is a gentle downward slope from 0 to 100 degrees Celsius.
When we talk about how much energy is in food, it’s important to understand that energy can take different forms. Take a carrot, for instance. Elevated atop a skyscraper, it has potential energy and, if released, could do serious damage to the sidewalk. A freshly baked carrot is full of thermal energy and could burn you. If we were to start a nuclear reaction with the carrot and manage to convert all its mass into energy, according to the famous equation from the theory of special relativity, , a big carrot could power the whole world for half a day.
So the real question we should be asking is: how do our bodies harvest energy from food? They do this through a series of chemical reactions collectively known as metabolism, which we will go into in more detail later. For now, it suffices to know that these reactions start with food and oxygen and end with carbon dioxide, water, indigestible waste, and energy. If we think of food as fuel, and the indigestible waste as ash, that sounds just like combustion. Actually, a calorimeter is the commonly used scientific tool to measure the energy yield of food upon its combustion. It consists of a pressurized capsule with food and pure oxygen inside, surrounded by water. You light the food on fire. After all the content is burned up, you measure how much the water’s temperature changed to figure out how much energy was released. On average, according to the calorimeter, carbohydrates release 4.2 kcal per gram, fats give off 9.5 kcal per gram, and proteins provide 5.65 kcal per gram.
However, if you look up calories for different foods in the USDA (U.S. Department of Agriculture) database, you will see that they use something called the Atwater General Factors: 4 kcal for each gram of carbohydrate, 9 kcal for each gram of fat, and 4 kcal for each gram of protein. The discrepancy between these figures and those obtained via calorimetry exists not because the government employs a bunch of elitists who think the general public cannot handle decimal points. Rather, it reflects the meticulous and considerate approach of Mr. Atwater in his investigations.
Let’s consider how the chemical energy is released differently in the body than in a calorimeter. First off, our bodies don’t digest everything we eat. Some stuff leaves our body as feces, and the energy in them is gone. Secondly, while a lab calorimeter can burn up all the nitrogen in proteins, we don’t. Excess nitrogen is converted to urea and leaves the body in urine. Third, the digestive process itself expends energy. Wilbur Atwater, the progenitor of the Atwater system and the inaugural chief of nutrition investigations at the USDA, arrived at those round numbers (4, 9, 4) after rigorously designed, conducted, and documented experiments. Atwater also determined the energetic value of alcohol to be 7 kcal per gram. That got him into trouble with his employer, Wesleyan University, under the patronage of the Methodist Church. This was not long before the Prohibition period. The Church was advertising that alcohol was nothing but poison. Atwater’s rebuttal laid bare the intrinsic contradictions of organized religions: the Almighty would not wish moral teachings to be based on untruths.
But the discrepancies between the available energy in food and the values measured in the calorimeter didn’t stop there. In the 1950s, USDA scientists concluded that the Atwater general factors should be further refined because: 1. There are 20 different amino acids, the building blocks of protein. Each one has its own chemical makeup. This means the amount of nitrogen can vary depending on the types of protein in the food. 2. The digestion of foods is influenced by their constituent mix, leading to differential absorption rates. The scientists crunched the data and built a big table for a list of foods. These are called the Atwater specific values. Here are some examples:
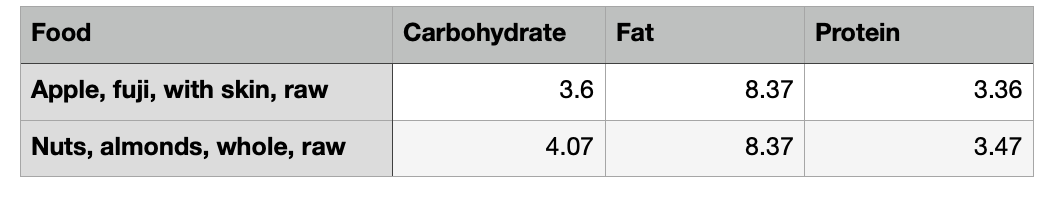
Now we are really collecting stamps.
But here’s the thing: we don’t actually get all the energy from the food we digest. Like digestion, the process of metabolism itself incurs an energy expenditure. Conversely, not all the energy in food we can’t digest is a total loss. Bacteria in the large intestine ferment certain fibers and produce free fatty acids, which are absorbed into the bloodstream. In light of these and other conditions, food scientist Geoffrey Livesey proposed the concept of net metabolizable energy. His numbers are: 3.2 kcal for protein, 8.9 kcal for fat, 3.8 kcal for available carbs, and 1.9 kcal for fermentable carbs.
Here is a brief summary of what we have learned so far.
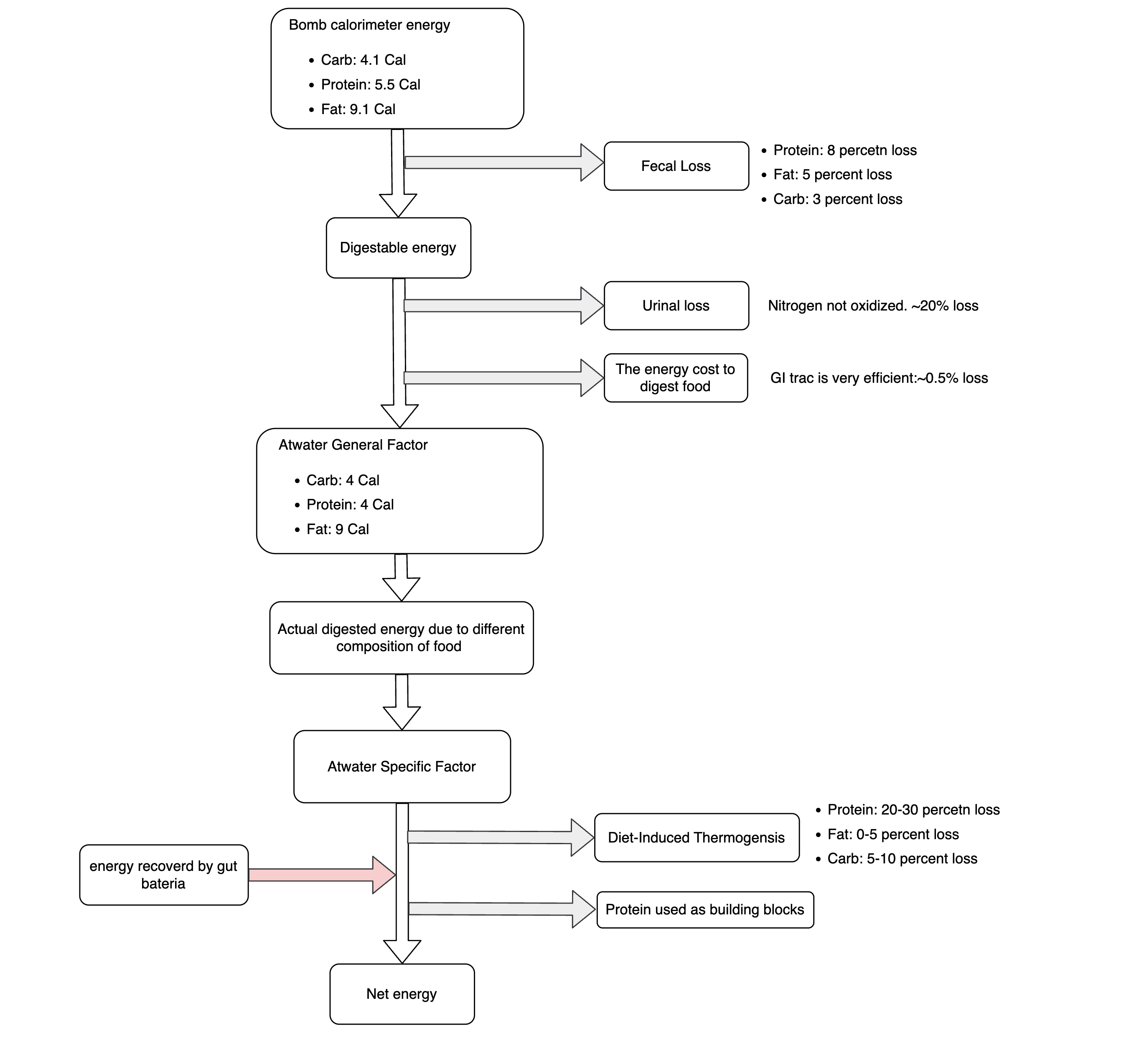
Figure 1. The flow of calories
Regrettably, we have not considered all the adjustments necessary to count calories accurately. For instance, almonds are pretty high in fat, but that fat is locked up inside cell walls that our bodies can’t break down. The size of these tiny cells is about 35 micrometers. After chewing, only about 10% of these cells are broken. Studies show that 30% fewer calories are absorbed from whole nuts compared to what’s available. The same almonds, if consumed in the form of slices, or if you put them in a blender and make almond butter, have more broken cells and consequently, unleash more fat in your body. For cooked food, it’s even more complicated, as discussed here.
I will end this discussion with a bit of good news for those of you counting calories: practically, the differences don’t matter. Even in the most carefully controlled studies, the error in weighing foods is rarely under 5%. As I mentioned earlier, how foods are prepared can significantly alter the amount of calories in them. Whatever inaccuracies exist in the Atwater factors, they are overwhelmed by these real-world variables. It’s not just my opinion. In 2002, the United Nations’ Food and Agriculture Organization (FAO) convened a meeting of international experts, including Livesey, to consider if it makes sense to use net metabolizable energy in place of the Atwater general factors. The conclusion? Don’t bother.